This is an opinion piece submitted to #MEAction and does not necessarily reflect the views of #MEAction. It should be considered as medical theory for discussion.
Could ME/CFS be the post-SIRS “immune paralysis” state of Compensatory Anti-Inflammatory Response Syndrome (CARS)? Or Mixed Antagonists Response Syndrome (MARS)?
The evidence we have about ME/CFS and the striking similarities between the illnesses provide a compelling argument that ME/CFS is a form of Systemic Inflammatory Response Syndrome (SIRS) as evidenced by a recent gene expression study which has so far revealed the disease as the closest resemblance to ME/CFS.
Further analysis of mRNA gene expression, in a study led by Dr. Lipkin and Dr. Mady Hornig, showed that the disease with the closest resemblance to ME/CFS is SIRS.
If this theory can be proven it would have enormous implications for funding ME/CFS research by casting a wider net to seek treatment for critically ill patients, not only for ME/CFS but for complications arising from concussion, burns, surgery, organ replacements and serious infections such as Pneumonia. The very syndromes only discussed in Surgical ICU’s that have challenged physicians for over 40 years could be the very same syndrome that has challenged ME/CFS practitioners all this time.
~
Twenty-five years ago in 1992, the American College of Chest Physicians and Society of Critical Care Medicine convened to address the confusion over the proper use of the terms and definitions for sepsis, where the use of the terms bacteremia, septicemia, sepsis, sepsis syndrome and septic shock were being used almost interchangeably. They agreed to a new set of definitions applied to patients in different stages of sepsis: bacteremia, SIRS, sepsis, severe sepsis, septic shock and Multiple Organ Dysfunction Syndrome (MODS). They also proposed to add the Compensatory Anti-Inflammatory Response Syndrome (CARS), and Mixed Antagonists Response Syndrome (MARS) to the set of clinical definitions, as the massive inflammatory reactions understood of SIRS and MODS was only half the picture.
It had become clear that quite rapidly after the first pro-inflammatory mediators are released, the body mounts a compensatory anti-inflammatory reaction as large as or larger than the pro-inflammatory response endeavoring to down-regulate the mediators and restore homeostasis. However, the overlapping processes these mediators initiate influence the endothelium, cardiovascular, hemodynamic and coagulation mechanisms, and
 the duration of the illness may alter the mix of mediators inducing metabolic disorders resulting in the body having no control over its own inflammatory response, an immunologic dissonance represented in the mnemonic by RC Bone et al as ‘CHAOS’. The ‘S’ stands for Suppression of the immune system where CARS predominates and what some investigators at the time called ‘immune paralysis’ – could this state of anergy be ME/CFS?
the duration of the illness may alter the mix of mediators inducing metabolic disorders resulting in the body having no control over its own inflammatory response, an immunologic dissonance represented in the mnemonic by RC Bone et al as ‘CHAOS’. The ‘S’ stands for Suppression of the immune system where CARS predominates and what some investigators at the time called ‘immune paralysis’ – could this state of anergy be ME/CFS?
The first acute immune reaction phase Systemic Inflammatory Response Syndrome (SIRS) is described as a subset of a cytokine storm or cytokine dysregulation, an inflammatory state with both pro- and anti-inflammatory components affecting the whole body, frequently a response of the immune system to infectious or non-infectious insult. SIRS can be caused by ischemia, inflammation, trauma, surgery complications, infection or several insults combined. Nearly every ICU patient (sometimes reported greater than 90%) fits the SIRS criteria and it is considered a serious condition related to systemic inflammation, potentially leading to organ dysfunction or organ failure. The criteria for SIRS includes at least two of the following:
- Body temperature hypothermic less than 36°C (96.8°F), or fever greater than 38°C (100.4°F)
- Tachycardia (abnormal heart rate) greater than 90 bpm
- Tachypnea (high respiratory rate) greater than 20 breath per minute
- White blood cell count elevated (leucocytosis >12*109/l) or depressed (leucopenia <4*109/l)
Also observed:
- Hyperglycemia (blood glucose >6.66mmol/L [120mg/dL] in absence of diabetes mellitus
- Altered mental status
Severe SIRS is frequently complicated by failure of one or more organs (MODS), or organ systems including circulatory system, digestive system, respiratory system (ARDS), endocrine system, immune and lymphatic system, muscular and nervous system. Sepsis often accompanies SIRS and the two share very similar symptoms. In fact, before 2016 SIRS formed the criteria for sepsis, but this was subsequently abandoned because it was considered too sensitive and meant nearly all ICU patients had sepsis. A published survey [Rangel-Fausto et al] revealed 48% of patients with SIRS progressed to developing sepsis within 28 days of admission.
Sepsis, which is derived from the Greek word “sepo” (meaning “I rot”), is regarded as a life-threatening condition, and is caused by your body’s response to infection most commonly bacterial, but can be from fungi, virus or parasite. Sepsis develops when the immune system releases chemicals into the bloodstream to fight infection, however; they cause systemic inflammation throughout the body instead. Sepsis can be mild to severe or even septic shock inducing a state of hypotension. The criteria for sepsis now uses the qSOFA (Quick Sequential Organ Failure Assessment) score before graduating to the full SOFA assessment and includes two of the following:
- Low blood pressure
- High respiratory rate
- Altered mental status
Measuring ME/CFS with SIRS
Considering the criteria for SIRS, it’s easy to see that many ME/CFS patients might qualify for a SIRS diagnosis, and many might also meet the criteria for sepsis using the qSOFA score. However, there is an important difference in that SIRS criteria usually diagnosed in patients in ICU is assessed during or immediately after the onset of the condition whereas ME/CFS patients are diagnosed after 6 months of their illness. It is fair to assume a few severe SIRS patients and certainly many Sepsis patients may not survive 6 months if it wasn’t for medical intervention, but what about a patient with mild SIRS and even perhaps mild sepsis with symptoms only just missing the diagnosis criteria and without any identifiable or treatable pathogen? There are after all no biomarkers yet for SIRS, Sepsis or ME/CFS, and all three are diagnosed by symptomatic observation. The estimated mortality rate of diagnosed SIRS is 6-7%, although a lower mortality rate is observed in patients without an identified infection. It has been estimated the mortality rate for ME/CFS is around 3-5%, most of these deaths are recorded as other causes.
Unfortunately, little early medical data of ME/CFS patients exists to measure against the SIRS criteria until they are diagnosed after 6 months of illness. There is, however, this own author’s own data which is worth noting:
“Within 50 days of my ME/CFS onset my white blood cell (WBC) was 3.9 and Neutrophils 1.7, indicating mild leukopenia and mild neutropenia. My renal function eGFR test of 71 (normal 90+) indicated mild kidney damage warranting further testing. I had great difficulty keeping warm indicating a constantly low body temperature and has subsequently ranged between high 35’s and mid 36’s. My ANA titre antinuclear antibodies at 320 were elevated (normal <160). My mental status was without doubt altered, even the simplest decisions seemed impossible to make. If I were in Hospital ICU at that time, I would have met the criteria for SIRS diagnosis (and possibly Sepsis). Within 6 months of becoming ill I was diagnosed with ME/CFS, but by then I would not have met the criteria for SIRS as my WBC, neutrophils, ANA and eGFR tests had all returned to within or near normal levels.”
“The micro-organisms that seem to have it in for us turn out to be rather more like
bystanders. It is our response to their presence that makes the disease. Our arsenal for fighting off bacteria are so powerful that we are more in danger from them than the invaders.” – Lewis Thomas
The inflammatory cascade of SIRS is often initiated by endotoxin or exotoxin. Tissue macrophages, monocytes, mast cells, platelets and endothelial cells are able to produce a multitude of cytokines. TNF-α and IL-1 are released first and initiate several cascades. With the NF-kB inhibitor removed, NF-kB is able to initiate the production of mRNA which induces production of other pro-inflammatory cytokines. TNF-α and IL-1 have been shown to be released in large quantities within 1 hour of insult, and these two cytokines together cause severe lung injury, hypotension, and cause fever and the release of stress hormones. It is understood the pro-inflammatory interleukins either directly or indirectly activate the coagulation cascade and the complement cascade and the release of NO, platelet activating factor, prostaglandins and leukotrienes. If unchecked this coagulation cascade, of which the complement system also plays a part, leads to complications of microvascular thrombosis and possibly organ dysfunction. At this unbalanced stage of the inflammation cascade, inflammation and coagulation dominate, both consistent with ME/CFS.
Compensatory Anti-inflammatory Response Syndrome (CARS) is the process by which the body is equipped to counter the inflammatory SIRS response thereby avoiding continuous injury to the host much like other homeostatic processes. The timely arrival of anti-inflammatory responses such as CARS seeks to limit the damage while not interfering with the pathogen elimination, however the response can be dangerous if poorly timed or its effects go unchecked, leaving the host vulnerable to excessive immunosuppression, and the next set of pathogens. The Cars response has been characterized to include:
- Cutaneous energy
- Reduction of lymphocytes by means of apoptosis
- Decreased cytokine response of monocytes to stimulation
- Decreased numbers of human leukocyte antigen (HLA) antigen-presenting receptors on monocytes
- Expression of cytokines such as IL-10 that suppress TNF expression.
Release of IL-4 and IL-10 are cytokines responsible for decreasing the production of TNF-α, IL-1, IL-6, and IL-8. The acute phase response in SIRS also produces antagonists to TNF-α and IL-1 receptors, either inactivating or blocking the cytokines. Normally a major characteristic of the CARS response is a Th1 – Th2 switch, however recent studies in post sepsis and peritonitis patients have observed in some patients down regulation of both Th1 and Th2 responses, suggesting a complete down regulation rather than a shift to an anti-inflammatory response. Further findings include down regulation of monocyte functioning, lower Dendritic cells and increased immune effector cell apoptosis. Reactivation of latent viruses is extremely common in patients with prolonged sepsis, consistent with development of immunosuppression comparable to organ transplant patients. Detection of CMV, EBV, HSV-1, and HHV-6 polyomaviruses (JC & BK) and anellovirus (TTV) occurred with high frequency in sepsis. A current hypothesis is that CMV and HSV reactivation may amplify sepsis induced lung and systemic inflammation thereby contributing to MODS. Additionally, chronic viral infections lead to T cell exhaustion and impaired immunity leads to further viral reactivation. An interesting finding very relevant to ME/CFS is a decreased mortality in septic patients with EBV viremia in blood (but not plasma) compared to EBV-neg patients. Research revealed that mice with low level gammaherpes-virus-68 infection (a murine virus genetically similar to human EBV) have improved survival by activating NK cells to produce IFN-γ, an essential factor for viral control. EBV is commonly reactivated in ME/CFS.
Contrary to earlier thinking that SIRS was the leading cause of sepsis mortality, it has since been established that late mortality has been typically associated with increased levels of anti-inflammatory cytokines associated with CARS and susceptibility to secondary complications.
Mixed Antagonist Response Syndrome (MARS), an immunological dissonance where at times there may be surges of hyperactivity (SIRS) and immunosuppression (CARS) which can become increasingly destructive and if severe enough leads to MODS, septic shock or a state of anergy. An uncorrected escalating deviation in either direction may result in death. A 2012 study on sepsis-induced mice by Osuchowski et al demonstrated that concurrent release of pro-inflammatory and anti-inflammatory cytokines occurs irrespective of the sepsis phase, severity or outcome. In that study, the progression of sepsis was monitored which revealed the lethal outcome in sepsis is not caused by a single mediator but is likely driven by concurrent deregulation of numerous immuno-inflammatory pathways.

And finally, a state of immune paralysis ensues as Persistent Inflammation, Immunosuppression and Catabolism Syndrome (PICS). Introduced in 2012 with improving management of SIRS-CARS response in surgical ICU’s and the resulting drop in MODS mortalities, an increasing number of patients were residing in ICU’s for weeks with a syndrome of manageable organ dysfunction, poor nutritional status, poor wound healing and recurrent infections. Additionally, they have persistent low grade inflammation with defects in innate and adaptive immunity including macrophage paralysis, persistent MDSC populations, and decreased effector T cell number and function. These patients are ultimately discharged to long term acute care facilities, rarely rehabilitate or return to functional life and usually suffer a prolonged decline and indolent death.

- Prolonged hospitalisation >14 days (Persistence)
- C-Reactive protein >150µg/dl (Inflammation)
- Total lymphocyte count <800/mm³ (Immunosuppression)
- Weight loss >10% during hospitalisation or BMI <18.
Creatinine Height Index <80%
Albumin <3.0gm/dl
Pre-albumin <10mg/dl
Retinol binding protein <10µg/dl (Catabolism)
Unfortunately, when PICS is recognised, rehabilitation of these patients into functional state is rare. The persistent inflammatory and catabolic state produces a ‘cachexia’ phenotype for which current ICU interventions are ineffective. PICS is extremely difficult to treat, there is currently no existing literature for nutritional support but one of the uniting characteristics of these patients is profound malnutrition which is refractory to any nutritional supplementation. It has been defined as similar to ageing sarcopenia, major burns, and cancer cachexia. Key challenges are now to identify PICS early, understand its underlying pathophysiology and apply multi-modal therapies that target specific components of the syndrome. It has been recognised that the incidence of PICS is likely to increase as our population ages and technologies improve, the recent estimate to 2025 is 80% increase. The University of Florida, Health Sepsis and Critical Illness Research Centre will be investigating the genomic makeup of PICS through a recently awarded grant by NIGMS entitled “PICS: A new horizon for surgical critical care”.
Post Sepsis Syndrome (PSS) is the term used to diagnose long term problems suffered by up to 50% of post sepsis patients. The symptoms of PSS include:

Charitable organisations and trusts have been established to care for these post-sepsis survivors. Surviving sepsis does not mean troubles are over for older adults, who face substantial cognitive impairment and functional disability afterward according to results from a longitudinal population based study. Researchers revealed the declines persisted for at least eight years.
The ME/CFS Sepsis comparison
ME/CFS is often associated with the Indian parable ‘the blind men and the elephant’ and has been called the ‘Disease of a Thousand Names’ as practitioner’s grapple with trying to understand the  cause of the disease. These names include: Neuromyasthenia, Chronic Mononeucleosis Syndrome, Atypical Poliomyelitis, Post Viral Fatigue, Epidemic Vasculitis, Myalgic Encephalomyelitis and Chronic Fatigue Syndrome. With more than five syndromes associated with sepsis, it seems the pathogenesis of sepsis has been equally difficult to determine and the exact humoral inflammatory mechanisms attributable to sepsis outcomes remain elusive.
cause of the disease. These names include: Neuromyasthenia, Chronic Mononeucleosis Syndrome, Atypical Poliomyelitis, Post Viral Fatigue, Epidemic Vasculitis, Myalgic Encephalomyelitis and Chronic Fatigue Syndrome. With more than five syndromes associated with sepsis, it seems the pathogenesis of sepsis has been equally difficult to determine and the exact humoral inflammatory mechanisms attributable to sepsis outcomes remain elusive.
Fundamental to the ME/CFS energy crisis is mitochondrial dysfunction. Recent research reveals a dysregulation or inhibition of the pyruvate dehydrogenase (PDH) enzyme as a key factor in ME/CFS pathogenesis. In sepsis, recent research has also pointed to cellular utilisation of oxygen that may be impaired (rather than oxygen delivery), where rodent models post-sepsis revealed inhibition of mitochondrial respiration. The ‘cytopathic hypoxia’ hypothesis in sepsis proposes that inflammatory mediators have a direct effect on the ability of the mitochondrion to utilise oxygen effectively, which can result in hibernation or cell death. Hibernation, or more accurately ‘dauer’, is precisely the term coined by a recent metabolomics study of ME/CFS patients by Dr Naviaux in association with Open Medicine Foundation. Altered metabolism within the cell is also demonstrated in sepsis by down regulation of the PDH enzyme leading to a decrease in the supply of energy to the cell.
The alternative sepsis hypothesis is the concept of Microcirculatory and Mitochondrial Distress Syndrome (MMDS). In sepsis, one of the critical steps may be an alteration in the flow through microcirculation, which is regulated by myogenic pressure, neurohormonal, and metabolic factors such as CO₂, lactate, and O₂ to meet oxygen demand of the cell. These autoregulatory mechanisms are disrupted in sepsis resulting in a mismatch between the requirements of the tissue for oxygen and its availability. Furthermore, the inducible form of nitric oxide synthase (iNOS) is switched on in response to inflammatory mediators, causing shunting of flow to those areas with there is most NO. Coupled to the activation of the coagulation pathway and red cell sequestration, the net effect is MMDS. Administration of fluids for resuscitation in the early stages of sepsis has demonstrated improved microcirculatory parameters shown up in new imaging techniques. Administration of fluids due to low blood volume in ME/CFS patients has also offered temporary remission (2-3 days), and there is at least one case of early administration of fluids resulted in complete remission.
The microcirculatory dysfunction in sepsis is considered to be an arginine deficient state. The endothelium is a single cell layer lining the inside of blood vessels and plays a central role in controlling the normal function of microcirculation. Sepsis inflammatory induced disturbance in cell-to-cell signalling causes the endothelial cells to become less responsive to vasoactive agents and lose their normal tight junction which results in capillary leakage. An arginine deficiency has been associated with the process, suggested to be the result of a decreased arginine uptake and an impaired synthesis from citrulline, in combination with arginine catabolism and inflammatory inducible nitric oxide synthase in the immune response. Gaseous nitric oxide is an important cellular signalling molecule, and helps modulate vascular tone, insulin secretion, airway tone, and peristalsis. Decreased arginine availability is believed to play a role in the downregulation of endothelial nitric oxide synthase (eNOS) and thereby in the decreased bioavailability of NO resulting in endothelial dysfunction. Furthermore, it is suggested the arginine depletion by competing iNOS and eNOS pathways can result in impaired T-cell function, not as a result of too much NO production but maldistribution in the immune response. Sepsis research has established maintaining arginine availability during inflammatory conditions is of crucial importance to enhance the arginine NO pathway. In 2015, Fluge & Mella applied for a patent for NO treatment for ME/CFS, the NO donor ‘surprisingly allow a treatment for ME/CFS patients for immediate relief of symptoms’ and an alternative strategy using supplemental l-arginine and l-citrulline. This affirms ME/CFS suffers the same microcirculatory dysfunction as sepsis.
The concept of MMDS is considered a model for describing sepsis and resuscitation and its role in the pathogenesis of MODS.
It has been suggested that what starts out as microcirculatory failure in early sepsis develops into mitochondrial dysfunction in late sepsis.

A Common Conundrum?
In ME/CFS and sepsis, all cells in the body experience a certain degree of stress through systemic inflammatory mediators, metabolic changes and stress hormones such as cortisol and catecholamines. This may lead to MODS. It appears though that the cells are able to adopt a state of ‘hibernation’. Down-regulating energy consumption maintains the ‘normal’ intracellular store of ATP, therefore allowing full recovery of cells that would be very sensitive to hypoxic injury. In sepsis research, this observation has led to the proposal that MODS is, potentially, a protective mechanism to preserve cellular integrity during stressful times. The ability of the mitochondrion to divide, increasing its numbers per cell, allows sufficient levels of ATP when recovering from this hibernating state. The consequences of this protective mechanism of course, if severe enough as seen often in intensive care, may be death.
The therapeutic conundrum asked both in sepsis and in ME/CFS research, if MODS or ME/CFS is a protective mechanism in the face of overwhelming sepsis or systemic inflammation, should we target therapy to improve mitochondrial function or instead first target support to failing organs and address symptoms awaiting resolution of the initial insult?
Could ME/CFS be Sepsis?
The sepsis illness concept is predicated on infection as its trigger and ME/CFS has not yet revealed an identifiable pathogen. However, in 1992 the sepsis definition (named ‘sepsis-1’) accommodated the patient’s systemic inflammation response and no definable bacterial infection was required. Another revision in 2001 (‘sepsis-2’) shifted focus to mortality risk, then in 2016 the current revision (‘sepsis-3’) is focused more on organ dysfunction proposing sepsis as “life threatening organ dysfunction due to a dysregulated host response to infection”. This latest revision is surrounded in controversy and has not been universally adopted. It seems no-one can agree on exactly what sepsis is, so it is unlikely ME/CFS will include the name sepsis any time soon. Nevertheless, researchers revealed that ME/CFS is identical to SIRS, a precondition to sepsis and formerly the criteria for sepsis. Furthermore, the paths of research for both ME/CFS and sepsis are converging, or have converged.
So, it seems with some certainty that ME/CFS, SIRS and Sepsis are all manifestations of systemic inflammation of varying severity and risk of MODS, separated perhaps only by the presence of an infection, sharing a common pathophysiology.

Systemic Inflammation / Sepsis Pathophysiology – a catastrophic cascade
Sepsis has been referred to as a process of malignant intravascular inflammation. While it begins as a typical inflammatory response to an infection, instead of winding down the septic reaction continues in an exaggerated, unregulated, and consequently destructive process. Sepsis is characterised by changes in the function of endothelial tissue which lines the inner surface of blood vessels, and changes in coagulation and blood flow. The excessive activation of coagulation factors and endothelial damage impedes blood flow particularly in micro-vessels and causes vessels to become permeable, fluid escapes into surrounding tissues which begin to swell. In the lungs this is called pulmonary edema which manifests as shortness of breath as the lung becomes heavy and poorly compliant, gas exchange is compromised and can lead to Acute Respiratory Distress Syndrome (ARDS).
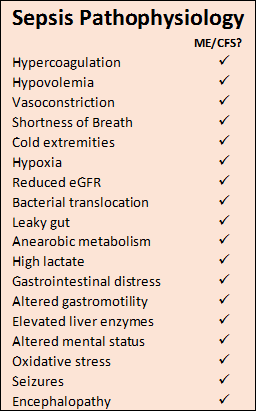
Leaky capillaries reduce blood volume and lowers blood pressure, and the vascular system responds with vasoconstriction to help the heart maintain cardiac output. Cold extremities are characteristic of this process. As sepsis progresses the heart muscle weakens due to circulating inflammatory molecules, the heart’s lower pumping power is compensated by dilated ventricles again assisting to maintain constant cardiac output. Like the lung, the kidney can become under-perfused and hypoxic, this dysfunction appears as a reduced glomerular filtration rate (eGFR) and an increase in serum creatinine. If sepsis continues, acute tubular necrosis develops leading eventually to acute renal failure.
Hypoperfusion limits supply of oxygen to the intestine, and as aerobic metabolism is superseded by anaerobic metabolism, lactate levels build in the portal vessels and the pH level drops inside the gut. This hypoxia and acidosis stress the epithelium that lines the gastrointestinal tract, and its natural barrier functions are weakened including protection against gut microbiota. Bacteria and toxic molecules from the gut lumen leak through the gut wall into the blood stream and lymphatics (bacterial translocation). Gastrointestinal distress and erosions in the upper GI mucosa are typical. Hypoperfusion slows the intestinal motility which can then develop paralytic ileus. The liver plays a key role in sepsis as a first line defence in the clearance of infectious agents, but sepsis-induced liver dysfunction by way of hemodynamic alterations leads to overrun of bacteria, toxins and debris into the circulation. Elevated liver enzymes and coagulations defects may occur, and a decreased ability to excrete toxins such as ammonia lead to encephalopathy.
Brain dysfunction caused by sepsis is characterised by fluctuating mental status, inattention and disorganised thinking known as septic encephalopathy. These changes are the result of the pro- and anti-inflammatory processes which have significant effect on vulnerable areas of the brain and reticular activating system. The endothelium of the blood vessels along the blood brain barrier (BBB) become permeable, leaking inflammatory molecules and infiltrating white cells into neural tissue. Subsequent swelling (edema) and damming of cells around arterioles hinder the brain’s supply of oxygen and nutrients and removal of metabolic wastes, neurons shut down and cerebral functions slow. Permeability of the BBB is further exacerbated by overexpression of inducible nitric oxide synthase (iNOS) in endothelial cells. Metabolic disturbances, circulatory failure and oxidative stress can lead to Wernicke’s Encephalopathy (caused by thiamine deficiency) and acute brain dysfunction with potential for long term neurologic impairment.
Post Sepsis & ME/CFS: immune suppressive disorders
Both in patients and in experimental models, septic patients develop a sustained anti-inflammatory or immunosuppressive state that has been termed ‘immuno-paralysis’ which is manifested by an inability to eradicate the primary infection or the development of new secondary infections. This complete down regulation of both Th1 and Th2 responses as referred to earlier in the definition of CARS is consistent with long-term and severe ME/CFS patients, while the majority of patients with shorter duration of illness (less than 3 years) exhibit a Th2 dominant immune response and increased cytokine profile. This is certainly very characteristic of the post sepsis immuno-paralysis state of anergy where CARS dominates as proposed in the following illustration representing what could be the typical immune response pathogenesis of ME/CFS.

The immature B-Cell connection
ME/CFS affects young adults with a gender ratio 4:1 female to male. This is similar to the age / gender related predominance in autoimmune disorders: IBS, MS, SLE, Sjogren’s. A 2008-2012 Norwegian study of ME/CFS age pattern revealed a surge from age 10, a peak age of 35 tapering to age 55 with a 3:1 F-M ratio. Coupled with elderly B-cell lymphoma risk and recent favourable B-cell depletion trials with rituximab, it has been suggested that ME/CFS may be an autoimmune disease. It has also been suggested there exists a genetic predisposition to ME/CFS owing to familial occurrences, although this could equally be linked to common viral, microbiological, environmental and dietary history. These theories however do not harmonize with the occasional epidemic nature of ME/CFS.
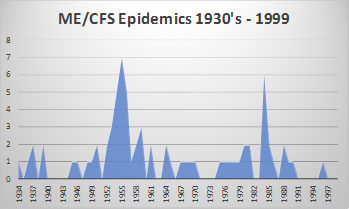 The infamous Royal Free Hospital ME/CFS epidemic in 1955 which spurned the ‘ME’ name, resulted in a hospital closing its doors for 10 weeks. In total, 292 members of 3500 medical staff (8.3%) were affected. The Royal Free Hospital group consisted of 5 hospitals and a medical school at the time, staff from all hospitals became ill. The hospital was filled with patients, yet strangely only 22 were affected. At the time, it was labelled ‘mass hysteria’ as no known cause was found. Since 1934, 62 epidemics of a similar disease have been described globally, reinforcing ME/CFS is a response to an infection. Similarly, the Gulf War Syndrome with ME/CFS like symptoms affected some 250,000 (35%) of the fit young soldiers who attended the 1991 Gulf War. There remain suspicions as to the cause of the infection (chemical warfare or their vaccines) but has otherwise remained elusive.
The infamous Royal Free Hospital ME/CFS epidemic in 1955 which spurned the ‘ME’ name, resulted in a hospital closing its doors for 10 weeks. In total, 292 members of 3500 medical staff (8.3%) were affected. The Royal Free Hospital group consisted of 5 hospitals and a medical school at the time, staff from all hospitals became ill. The hospital was filled with patients, yet strangely only 22 were affected. At the time, it was labelled ‘mass hysteria’ as no known cause was found. Since 1934, 62 epidemics of a similar disease have been described globally, reinforcing ME/CFS is a response to an infection. Similarly, the Gulf War Syndrome with ME/CFS like symptoms affected some 250,000 (35%) of the fit young soldiers who attended the 1991 Gulf War. There remain suspicions as to the cause of the infection (chemical warfare or their vaccines) but has otherwise remained elusive.
If ME/CFS is systemic inflammation, then there would likely have been outbreaks of SIRS-like illnesses. In 1918, the worst pandemic in human history, the H1N1 Spanish Flu infected 5% of the world’s population killing 2% (50-100 million), with a specifically high young adult mortality rate. The virus was controversially recreated in 2006 from a preserved frozen victim to transfect monkeys for observation, the symptoms exhibited widespread lung tissue damage within 24 hours. While typical influenza respiratory complications such as pneumonia were responsible for many deaths, there is compelling evidence of a good number of systemic inflammatory responses leading to ARDS. The Spanish Flu peak age mortality was 28 years, and notable for being atypically fatal to those aged 20-40 years. Mortality for men was higher but was likely affected by TB complications and male concentrations during WW1. Preliminary studies using mouse adapted H1N1 Flu revealed that females mount higher inflammatory responses, correspondingly TIV Flu vaccines for women are half the dose of men. Once a patient is diagnosed with sepsis, gender again influences outcome. In ICU, men aged 14-40 have a higher incidence of sepsis and a higher rate of mortality, for women aged 14-40 their ICU stay is typically longer and their survival rate significantly higher (74%) compared to men (31%). Over 50 years of age however, men trended toward marginally lower mortality rates than women.
So, what might be the susceptibility to ME/CFS and systemic inflammatory response in seemingly young healthy adults with a female predominance? So far, T Cell dysfunction has been discussed in the immune response, but it has recently been discovered B-Cells can enhance early innate immune responses. Influenza infection triggers a robust B cell response in the lymphoid tissue of the respiratory tract. Recently in sepsis models, innate like / marginal zone B cells have been shown to produce pro- and anti-inflammatory cytokines (IL-6, IL10) and exacerbate systemic inflammatory responses to endotoxic shock. In ME/CFS patients, there have been observed increased proportions of transitional and naïve B cells, and are suspected to be involved in the 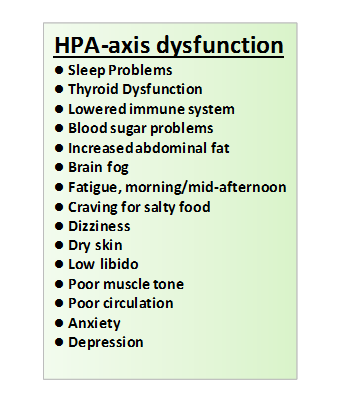 pathogenesis. It has been hypothesised that B-cell driven diseases (SLE, thyroiditis, and other diseases of younger age) reach their maximum incidence in the reproductive years during the period when higher sex hormones stimulate B cells, but inhibit T cells, macrophages and fibroblasts. In contrast, it is hypothesized that diseases in postmenopausal years are mainly driven by T cells, macrophages and or fibroblasts when the loss of sex hormones is conducive. Thus, we are all more vulnerable to B cell induced diseases during young adult life, but particularly women whose serum sex hormones are up to ten times higher than men (opposite for testosterone).
pathogenesis. It has been hypothesised that B-cell driven diseases (SLE, thyroiditis, and other diseases of younger age) reach their maximum incidence in the reproductive years during the period when higher sex hormones stimulate B cells, but inhibit T cells, macrophages and fibroblasts. In contrast, it is hypothesized that diseases in postmenopausal years are mainly driven by T cells, macrophages and or fibroblasts when the loss of sex hormones is conducive. Thus, we are all more vulnerable to B cell induced diseases during young adult life, but particularly women whose serum sex hormones are up to ten times higher than men (opposite for testosterone).
Endocrine Dysfunction in sepsis

In ME/CFS, HPA axis dysfunction has been studied with evidence supporting the presence of mild hypocortisolism, attenuated diurnal variation of cortisol, enhanced negative feedback to the HPA axis and blunted HPA axis responsiveness. Studies in systemic disease have shown continuously high levels of different cytokines can inhibit HPA axis function: ACTH and cortisol responses were nearly completely blunted after 3 weeks of IFN-α injection. How would they respond after a cocktail of different pro-inflammatory cytokines as is the situation of ongoing systemic inflammation? It has been hypothesized that the rapid rise and fall of ACTH/cortisol was evolutionary positive in order to support an early response but not a long term inhibitory influence on the immune system. Reasons suggested include:
- the initially elevated cortisol levels will rapidly inhibit hypothalamic neurons stopping CRH secretion (negative feedback regulation)
- prolonged increase of inflammatory stimuli with elevated TNF, IFN-α, or IL-6 serum levels will diminish HPA axis responsiveness
- longer term elevated pro-inflammatory cytokines influence the secretion of steroid hormones from the adrenal glands
- age related increase of serum IL-6 in healthy patients may reduce the HPA axis responsiveness due to continuous stimulation
- the cytokine-stimulated increase of cortisol secretion from the liver enhances the negative feedback toward the hypothalamus, leading to low ACTH and adrenal androgens, but normal or slightly elevated serum cortisol.
It has been suggested therefore that the HPA axis has an inherent defect, in the inability to mount an appropriately enhanced glucocorticoid response to increased secretion of pro-inflammatory cytokines. In a chronic inflammatory systemic disease such as sepsis, SIRS and ME/CFS, such an inadequate response can play a disease perpetuating role when cortisol secretion and its anti-inflammatory effectiveness is low.
In summary, it has been established that ME/CFS is a systemic inflammatory response closely resembling SIRS. History has shown that SIRS can occur in pandemics, with atypical age range common to ME/CFS suggesting susceptibility to an immune hyper-responsiveness possibly linked to B cells, sex hormones and other influencing factors. There are no biomarkers yet for SIRS or sepsis, differentiation between the two is still difficult to determine. Sepsis is referred to as malignant intravascular inflammation. Controversy still surrounds the sepsis definition that requires the presence of an infection and whether the possibility exists that ME/CFS is included within the sepsis syndrome in the future. There can, however, be no dispute that the two illnesses share not only the same research challenges, but the same pathogenesis, the same symptomology, the same responses to therapy and supplements, and the same uncertain prognosis.
It seems logical that the ME/CFS community should begin to share knowledge and resource with the sepsis organizations such as International Sepsis Forum (ISF), Global Sepsis Alliance and Sepsis Trust and Post Sepsis Syndrome patients. The curative challenges ahead are monumental, involving complex pathways which have yet to be researched, and will no doubt result in individualized treatments. Early recognition and intervention is the key to minimize the severity and outcome which will require global awareness and hopefully detection with the assistance of early biomarkers. It seems plausible that the HPA axis dysfunction could be a perpetuating factor in the disease and that adrenal insufficiency should be an early target for therapeutic research. Understanding the pathogenesis of ME/CFS will hopefully lead to the discovery of new supplemental symptom support within the remarkably stoic and resourceful ME/CFS community, which in sepsis critical care is targeted at anti-inflammatory and anti-oxidative activity.
Above all, ME/CFS deserves a new name in recognition as a systemic inflammatory disease: sepsis is estimated to affect 27m people per year worldwide, with 8m fatally, many survivors suffering long-term complications. The annual financial burden is estimated well in excess of US$200 billion.
Sepsis Treatment in Critical Care
Sepsis Treatment, like for ME/CFS is somewhat of a bare cupboard in critical care, no pharmacologic agents have been demonstrated to improve the outcome of SIRS. Only one drug has been approved for treating severe sepsis in adults: recombinant human activated protein C (rhAPC), or drotrecogin alfa (activated). However, this was withdrawn because it was targeted at the pro-inflammatory state of SIRS as the cause of sepsis with dismal results. It has since been recognised that effective correction should blunt hyperactive responses or boost suppressed responses, and the same drug may be beneficial, noneffective or even harmful depending on the patient’s immunological status. It is likely treatment will lie in an individual approach.
The initial early management of sepsis is critical and targeted at avoiding tissue hypoperfusion and hypoxia by optimising oxygen delivery. This involves aggressive fluid resuscitation, ionotropic, and vasoactive agents which can improve outcome.
Hyperglycemia is a common finding even in individuals without diabetes and has numerous deleterious systemic effects. Anti-diabetic agents used to treat hyperglycemia reduced in-hospital mortality rates by 34% by maintaining blood glucose at 80-110mg/dL. Presently, the surviving sepsis guidelines recommend keeping glucose levels at less than 180mg/dL.
Blood lactate are often measured in critically ill patients, thought to be indicators of anaerobic metabolism associated with tissue dysoxia. Lactate levels are commonly elevated from increased peripheral intraorgan production, reduced hepatic uptake, and reduced renal elimination. Numerous studies have found that lactate levels correlate strongly with mortality.
Corticosteroids showed a trend toward worse outcomes in sepsis and septic shock when treated with high doses; however, research into low dose steroids (200-300mg hydrocortisone for 5-7 days) improved survival and reversal of shock in vasopressor-dependent patients.
Supplemental adjunctive therapy in sepsis has targeted anti-oxidation and anti-inflammatory activity. Selenium concentrations have been shown to be lowest in patients with sepsis and correlate with poor outcomes. Due to intracellular anti-oxidant and pro-inflammatory inhibitory properties, selenium supplementation has been suggested to improve outcomes. A recent test of high bolus doses of selenase® significantly reduced mortality in sepsis by 27%, and maintenance therapy 7 days significantly improved survival. Zinc supplementation has also been associated with improved sepsis survival, and while comparative human tests have not been conducted several murine models have exhibited much improved outcomes. Zinc is involved in both innate and adaptive immune function, and serves important roles in oxidative stress response and neurocognitive function. Vitamin E has effects on inflammatory processes due to antioxidant functions of α-tocopherol, decreasing levels of pro-inflammatory cytokines and inhibiting protein kinase C activity. Higher doses can be counterproductive.





9 thoughts on “Is SIRS, CARS, MARS – and now PICS – causing the "CHAOS" in ME/CFS?”
I saved this article several years ago : https://www.ncbi.nlm.nih.gov/pubmed/10725796
Very pertinent, thanks Marlene. This helps answer my one nagging question why some descend into ME/CFS without a preceding insult (sympathetic activation of primary anti-inflammation).
Maybe it would be good to cite previous research on the role of CIRS in ME/CFS and depression (2013)
CIRS: conceptualized as a CARS but in mild chronic inflammatory disorders, incl ME/CFS
CIRS: compensatory anti-inflammatory reflex system
For ME/CFS see:
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3751187/
A narrative review on the similarities and dissimilarities between myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) and sickness behavior
It is of importance whether myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) is a variant of sickness behavior. The latter is induced by acute infections/injury being principally mediated through proinflammatory cytokines. Sickness is a beneficial behavioral response that serves to enhance recovery, conserves energy and plays a role in the resolution of inflammation. There are behavioral/symptomatic similarities (for example, fatigue, malaise, hyperalgesia) and dissimilarities (gastrointestinal symptoms, anorexia and weight loss) between sickness and ME/CFS. While sickness is an adaptive response induced by proinflammatory cytokines, ME/CFS is a chronic, disabling disorder, where the pathophysiology is related to activation of immunoinflammatory and oxidative pathways and autoimmune responses. While sickness behavior is a state of energy conservation, which plays a role in combating pathogens, ME/CFS is a chronic disease underpinned by a state of energy depletion. While sickness is an acute response to infection/injury, the trigger factors in ME/CFS are less well defined and encompass acute and chronic infections, as well as inflammatory or autoimmune diseases. It is concluded that sickness behavior and ME/CFS are two different conditions.
Robert what do you make of Dr Marik’s hydrocortisone, IV Vitamin C, IV thiamine protocol for SIRS as a treatment modality for ME?
From his 2017 study:
“RESULTS: There were 47 patients in both treatment and control groups, with no significant differences in baseline characteristics between the two groups. The hospital mortality was 8.5% (4 of 47) in the treatment group compared with 40.4% (19 of 47) in the control group (P < .001). The propensity adjusted odds of mortality in the patients treated with the vitamin C protocol was 0.13 (95% CI, 0.04-0.48; P = .002). The Sepsis-Related Organ Failure Assessment score decreased in all patients in the treatment group, with none developing progressive organ failure. All patients in the treatment group were weaned off vasopressors, a mean of 18.3 ± 9.8 h after starting treatment with the vitamin C protocol. The mean duration of vasopressor use was 54.9 ± 28.4 h in the control group (P < .001).
https://www.ncbi.nlm.nih.gov/m/pubmed/27940189/"
Hello Jesse2233, so sorry my late reply, I must have un-ticked notification of comments. I had observed Dr Marik’s treatment with interest, and it warrants further evaluation. As far as treatment for ME/CFS? I am not a doctor, but it is my researched opinion that ME/CFS if identified early enough would respond to the same treatment as SIRS/sepsis. Early enough may mean a window of weeks, but not after the 6 months ME/CFS diagnosis. In my current research there is good evidence of immune cell proliferation by the warburg effect in ME/CFS, even my own OAT results are identical to sepsis altered metabolism, and that ME/CFS is essentially (or evolves into) a cachexia state or critical illness polyneuropathy (CIP). I am exploring amino acid therapy, something which I believe will be a significant component of ME/CFS rehabilitation. I hope to have a new opinion piece within the next couple of months on all this, backed with good science. I’m excited by the prospects so far.
What about nanobacters? I think this research is on the right track.
Anything’s possible, and certainly gram-negative bacteria is in my current hypothesis due out this month. I’m more inclined to think LPS, the evidence is there. LPS induces M1 polarisation, which fits ME/CFS in my opinion, which contributes to sepsis Reg-T-cell immuno-suppression and also insulin resistance. LPS induces warburg effect metabolism with upregulated PKM2 isoform. Stress hyperglycemia is also part of the illness, with a higher glucose diffusion gradient, and de-novo fatty acid synthesis, ME/CFS becomes a pre-diabetic and dyslipidemic state, driving ER Stress and chronically immunosuppressed. Ketogenic reverses all this just as in cancer and shifts to M2 polarisation. It appears to be best therapeutic offer on the table at present. I feel confident my next article sums up ME/CFS, so keep a look out!
When will your article summing up ME/CFS be posted?
Hi MD, thanks for asking – it is still in construction, but I hope before end May to release.
Comments are closed.